TL;DR
- Many digital marketing tools collect PHI without consent, violating HIPAA.
- Retargeting via Meta Pixel and GA4 can expose sensitive user behavior.
- Covered entities must manage client-side risks like unauthorized trackers.
- Feroot provides real-time visibility into digital tracking for HIPAA compliance.
- Avoid fines by enforcing privacy rules across ad and analytics platforms.
Why Is Digital Marketing Risky Under HIPAA?
Digital marketing relies on user behavior data — but for healthcare organizations, that data often includes protected health information (PHI). If ad platforms or third-party scripts collect PHI without consent or encryption, your organization could face HIPAA violations.
Common marketing tools that can trigger HIPAA compliance issues:
- Google Analytics 4 (GA4)
- Meta Pixel (Facebook)
- Programmatic ad networks
- Email and SMS marketing platforms
- CRM integrations with web trackers
HIPAA enforcement agencies, including the OCR, have issued warnings and fines related to improper use of these tools, particularly when sensitive health-related pages (e.g., symptom checkers, appointment forms) are involved.
What Tracking Technologies Violate HIPAA?
HIPAA is technology-neutral, but violations occur when PHI is collected, stored, or shared without:
- Valid patient authorization
- A signed Business Associate Agreement (BAA)
- Proper encryption or anonymization
- Access logging and controls
Tracking technologies often responsible for violations:
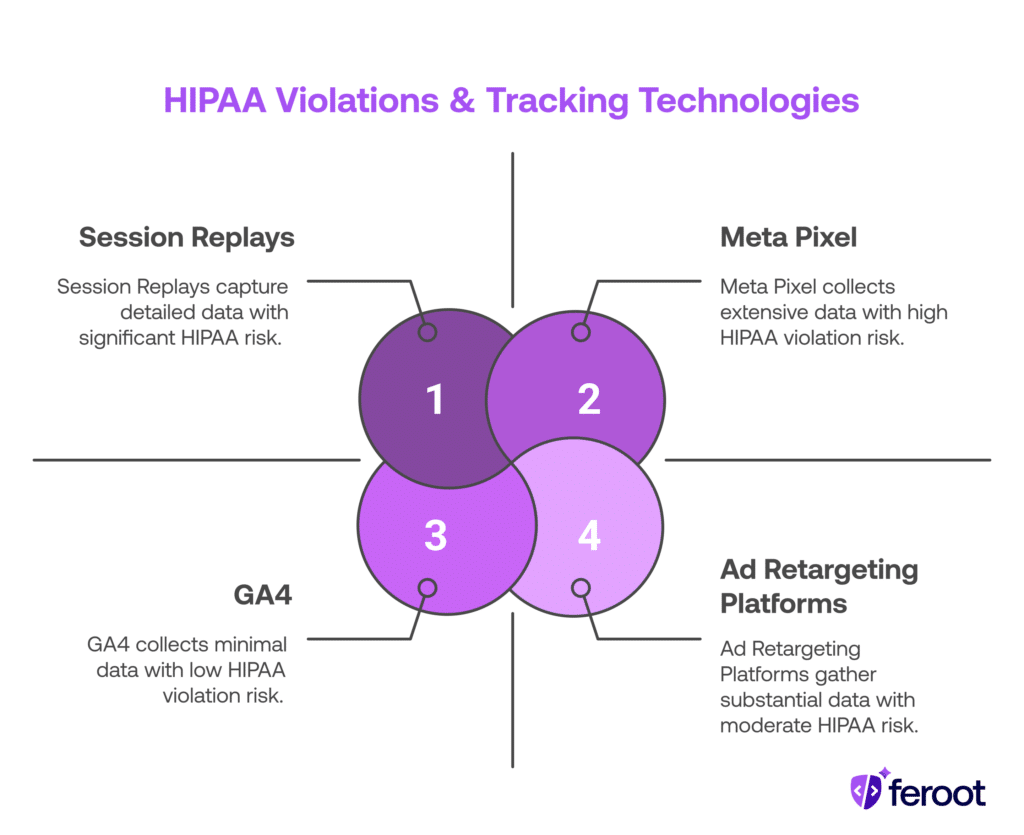
- Meta Pixel: Sends behavioral data back to Facebook, often without a BAA
- GA4: Collects event-based user data (e.g., page views, button clicks) that may reveal health interests
- Session replays: Tools like Hotjar or FullStory can capture typed information or appointment data
- Ad retargeting platforms: Re-engage users based on visit history, which may infer medical intent
What Are the Top 5 HIPAA Compliance Errors in Retargeting?
1. Using Tracking Pixels on PHI-Revealing Pages
If your website includes scheduling, symptom checkers, or treatment info — and Meta Pixel or GA4 is running — you’re likely transmitting PHI without consent or a BAA.
Example: A fertility clinic embedded Meta Pixel on their appointment page. Meta received data including the user’s IP, action timestamps, and page path — all PHI under HIPAA.
2. Retargeting Without Explicit Consent
Retargeting users based on prior site visits can violate HIPAA if their behavior implies health-related intent and you haven’t obtained explicit authorization.
3. Lack of Visibility into Client-Side Data Flows
Most teams don’t monitor what JavaScript or third-party scripts are actually doing in the browser. This blind spot leads to unauthorized data leaks via:
- Shadow scripts
- Inherited marketing tags
- Unauthorized third-party requests
4. Assuming a BAA Alone Guarantees Compliance
Even if your analytics vendor signs a BAA, you’re still responsible for ensuring PHI isn’t collected inappropriately. Misconfigured tags or over-broad data collection can still be violations.
5. Using Ad Platforms That Don’t Support HIPAA Compliance
Platforms like Meta, TikTok, and others publicly state they are not HIPAA-compliant. Using them for patient outreach or behavioral retargeting can result in noncompliance.
What Enforcement Actions Have Been Taken for HIPAA Marketing Violations?
OCR and FTC enforcement has intensified against healthcare organizations that share PHI with ad platforms through online tracking — especially without proper consent or safeguards.
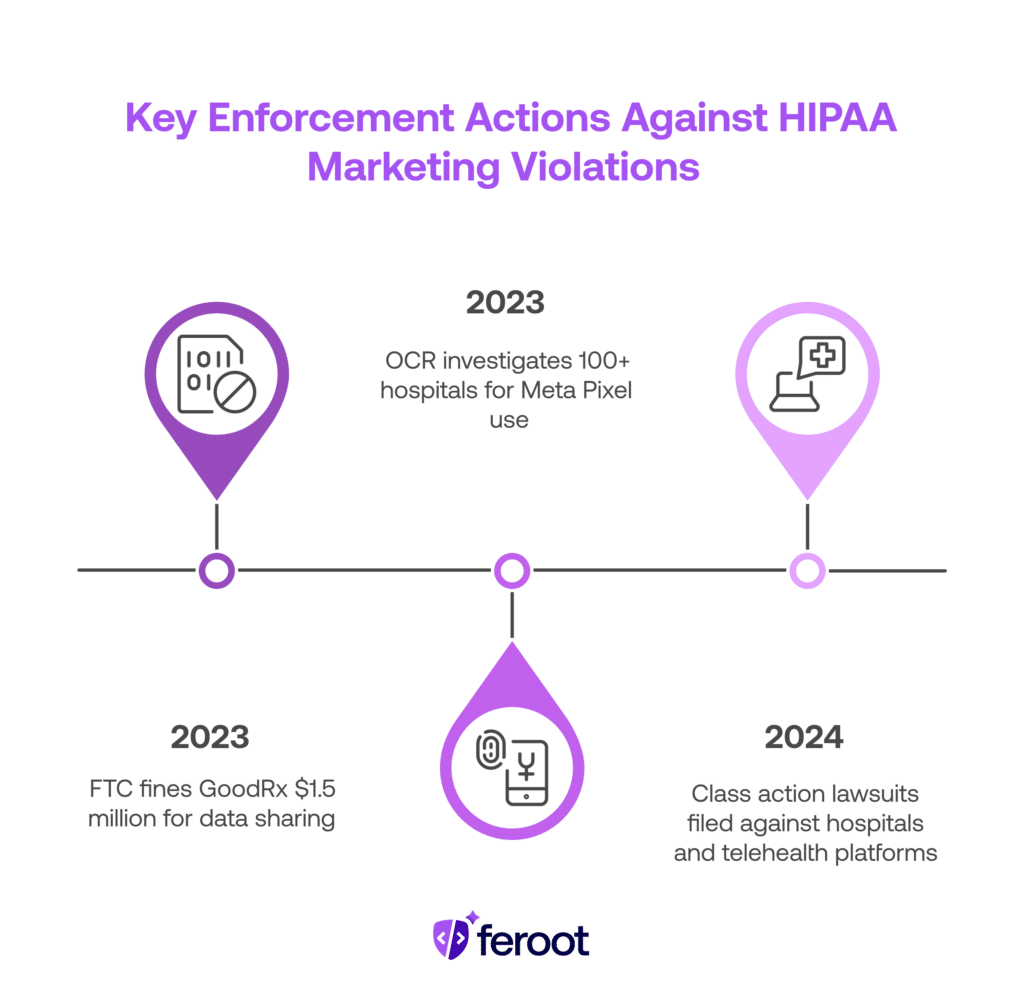
2023 – FTC fines GoodRx $1.5 million
GoodRx shared users’ health-related data with Facebook, Google, and others for retargeting. FTC ruled this violated both HIPAA and FTC Act protections.
2023 – OCR investigates 100+ hospitals
Following a report on Meta Pixel use, OCR launched probes into healthcare systems using tracking tech on appointment or portal pages.
2024 – Class action lawsuits filed
Patients sued hospitals and telehealth platforms over data leakage via marketing pixels. Settlements reached in some cases.
Takeaway: Enforcement agencies view retargeting and behavioral advertising through the HIPAA compliance lens — especially when patients have no idea their data is being shared.
How Does Feroot Help Healthcare Marketers Stay HIPAA-Compliant?
Feroot protects healthcare organizations from client-side privacy violations — especially those caused by third-party trackers and retargeting tools.
Why this matters:
Many HIPAA violations originate in the browser, not the backend. HIPAA compliance is jeopardized by tracking pixels, ad scripts, and third-party analytics tools run in users’ browsers — where PHI can be leaked silently.
What Feroot does:
- Monitors JavaScript and browser-side scripts in real time
- Flags unauthorized third-party data flows from sensitive pages
- Enforces security and privacy controls across digital properties
- Maps browser behavior to HIPAA privacy and security rules
- Generates audit-ready reports showing PHI protection across tools
What Can CISOs and Marketing Teams Do to Reduce Risk?
1. Audit all tracking tools running on PHI-relevant pages
Check for Meta Pixel, GA4, session replays, and tag manager scripts.
2. Disable behavioral retargeting for healthcare-specific journeys
Do not run ads based on symptom checker, form completion, or appointment pages.
3. Require explicit patient authorization for marketing campaigns
If PHI will be used, ensure it’s documented and permissioned.
4. Use platforms that offer HIPAA compliance configurations
Some email/SMS tools and analytics vendors offer scoped tracking or BAAs.
5. Implement real-time client-side monitoring
Tools like Feroot give you visibility into what’s actually happening in the user’s browser.
Conclusion
Healthcare marketing and HIPAA compliance are increasingly at odds — especially in a digital ecosystem filled with trackers, ad platforms, and behavioral analytics. But violations don’t have to be inevitable.
With the right visibility and controls, CISOs and marketing teams can:
- Avoid unintentional PHI exposure in retargeting
- Reduce risk from third-party tracking tools
- Prove HIPAA compliance with privacy and security rules
FAQ
Is it illegal to use Meta Pixel on a healthcare website?
Not always — but if it’s running on pages that handle PHI and you don’t have user authorization or a BAA, it likely violates HIPAA.
What counts as PHI in digital marketing?
Any user behavior tied to an individual and related to health conditions, services, or payments — including IP address, device ID, or clickstream data on treatment pages.
Can Google Analytics 4 be HIPAA-compliant?
No. Google states GA4 is not HIPAA-compliant and should not be used to collect or store PHI.
How can Feroot help with HIPAA audits?
Feroot provides audit-ready reports of client-side data activity, proving your organization is protecting PHI from unauthorized tracking or leakage.
Do healthcare marketers need to be HIPAA-trained?
Yes. Anyone handling campaigns, site tracking, or patient engagement should be trained in HIPAA basics — including digital risks.